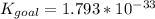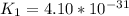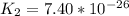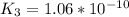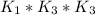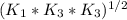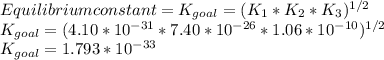Chemistry, 21.01.2020 06:31 joelpimentel
Determine the value of the equilibrium constant, kgoal, for the reactionn2(g)+h2o(g)⇌no(g)+12n2h4(g ), kgoal=? by making use of the following information: 1. n2(g)+o2(g)⇌2no(g), k1 = 4.10×10−312. n2(g)+2h2(g)⇌n2h4(g), k2 = 7.40×10−263. 2h2o(g)⇌2h2(g)+o2(g), k3 = 1.06×10−10express your answer numerically.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3
You know the right answer?
Determine the value of the equilibrium constant, kgoal, for the reactionn2(g)+h2o(g)⇌no(g)+12n2h4(g...
Questions

English, 26.05.2021 14:40

Chemistry, 26.05.2021 14:40


History, 26.05.2021 14:40


English, 26.05.2021 14:40

Social Studies, 26.05.2021 14:40


History, 26.05.2021 14:40


Chemistry, 26.05.2021 14:40


Mathematics, 26.05.2021 14:40