
Asolution is prepared by adding 49.3 ml concentrated hydrochloric acid and 19.5 ml concentrated nitric acid to 300 ml water. more water is added until the final volume is 1.00 l. calculate h and the ph for this solution. [hint: concentrated hcl is 38% hcl (by mass) and has a density of 1.19 g/ ml; concentrated hno3 is 70% hno3 (by mass) and has a density of 1.42 g/ml.]
[h+] =
oh- =
ph =

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1


Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
Asolution is prepared by adding 49.3 ml concentrated hydrochloric acid and 19.5 ml concentrated nitr...
Questions


English, 30.11.2019 02:31

Mathematics, 30.11.2019 02:31

Mathematics, 30.11.2019 02:31

Mathematics, 30.11.2019 02:31

Mathematics, 30.11.2019 02:31

Social Studies, 30.11.2019 02:31

Mathematics, 30.11.2019 02:31

Mathematics, 30.11.2019 02:31


Biology, 30.11.2019 02:31

Social Studies, 30.11.2019 02:31

Chemistry, 30.11.2019 02:31

Biology, 30.11.2019 02:31





Biology, 30.11.2019 02:31

Social Studies, 30.11.2019 02:31

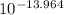 = [OH⁻] = 1.086x10⁻¹⁴ M
= [OH⁻] = 1.086x10⁻¹⁴ M

