
Chemistry, 21.01.2020 22:31 edgartorres5123
According to a label on a bottle of concentrated hydrochloric acid, the contents are 36.0% hcl by mass and have a density of 1.18 g/ml.
1. what is the molarity of concentrated hcl.
2. what volume of it would you need to prepare 985 ml of 1.6 m hcl?
3. what mass of sodium bicarbonate would be needed to neutralize the spill if a bottle containing 1.75 l of concentrated hcl dropped on a lab floor and broke open?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2


Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
According to a label on a bottle of concentrated hydrochloric acid, the contents are 36.0% hcl by ma...
Questions







Mathematics, 21.01.2020 04:31

Mathematics, 21.01.2020 04:31







Computers and Technology, 21.01.2020 04:31






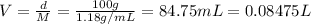
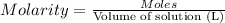
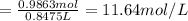
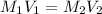 ( Dilution equation)
( Dilution equation)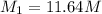
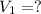
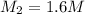
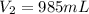
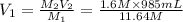
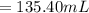
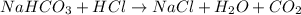
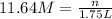
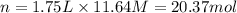
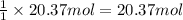 of sodium carbonate
of sodium carbonate 

