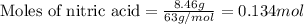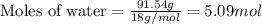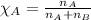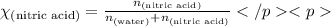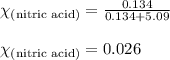Chemistry, 21.01.2020 23:31 sedilei1515
What is the mole fraction of nitric acid in a(n) 8.46% (by mass) aqueous solution of nitric acid

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1
You know the right answer?
What is the mole fraction of nitric acid in a(n) 8.46% (by mass) aqueous solution of nitric acid...
Questions




Computers and Technology, 27.12.2019 05:31




English, 27.12.2019 05:31



Mathematics, 27.12.2019 05:31

Mathematics, 27.12.2019 05:31

History, 27.12.2019 05:31

History, 27.12.2019 05:31




Mathematics, 27.12.2019 05:31

Biology, 27.12.2019 05:31

 .....(1)
.....(1)