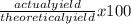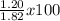Chemistry, 22.01.2020 00:31 jonmorton159
The actual yield of a product in a reaction was measured as 1.20 g. if the theoretical yield of the product for the reaction is 1.82 g, what is the percentage yield of the product?
a. 65.9%
b. 67.2%
c. 71.9%
d. 73.3%

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
The actual yield of a product in a reaction was measured as 1.20 g. if the theoretical yield of the...
Questions

Mathematics, 12.10.2020 21:01





History, 12.10.2020 21:01

History, 12.10.2020 21:01


Mathematics, 12.10.2020 21:01






Computers and Technology, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01

French, 12.10.2020 21:01

English, 12.10.2020 21:01