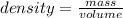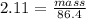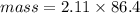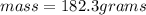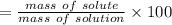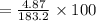Chemistry, 22.01.2020 04:31 ayoismeisjjjjuan
Asolution is made by dissolving 4.87 g of potassium nitrate in water to a final volume of 86.4 ml solution. what is the weight/weight % or percent by mass of the solute? (i got 5.64% but it said it was incorrect, and i can't figure it out? )

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
Asolution is made by dissolving 4.87 g of potassium nitrate in water to a final volume of 86.4 ml so...
Questions


Mathematics, 12.05.2021 20:50


History, 12.05.2021 20:50



Mathematics, 12.05.2021 20:50


Mathematics, 12.05.2021 20:50

Mathematics, 12.05.2021 20:50

Mathematics, 12.05.2021 20:50


Mathematics, 12.05.2021 20:50

Geography, 12.05.2021 20:50


Social Studies, 12.05.2021 20:50

Business, 12.05.2021 20:50

Physics, 12.05.2021 20:50


Mathematics, 12.05.2021 20:50