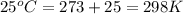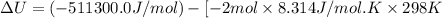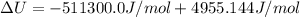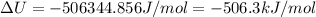Chemistry, 22.01.2020 04:31 george6871
The standard internal energy change for a reaction can be symbolized as δ u ∘ rxn or δ e ∘ rxn . for each reaction equation, calculate the energy change of the reaction at 25 ∘ c and 1.00 bar . sn ( s ) + 2 cl 2 ( g ) ⟶ sncl 4 ( l ) δ h ∘ rxn = − 511.3 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
The standard internal energy change for a reaction can be symbolized as δ u ∘ rxn or δ e ∘ rxn . for...
Questions


Mathematics, 28.06.2021 21:40


Mathematics, 28.06.2021 21:40

Mathematics, 28.06.2021 21:40





Computers and Technology, 28.06.2021 21:40




Physics, 28.06.2021 21:40






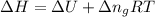
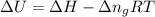
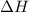 = change in enthalpy =
= change in enthalpy = 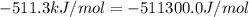
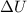 = change in internal energy = ?
= change in internal energy = ?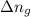 = change in moles
= change in moles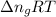 = 0
= 0