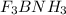Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

Chemistry, 23.06.2019 09:00
How many moles of sulfur dioxide are in 2.26 × 10^33 sulfur dioxide molecules?
Answers: 3
You know the right answer?
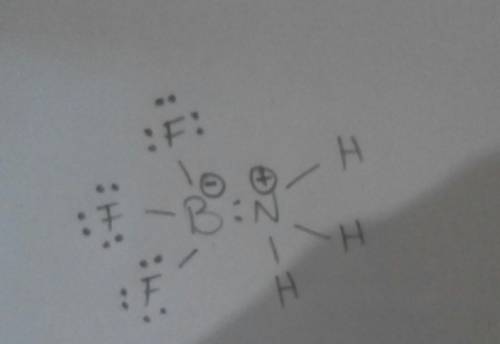
Write the lewis structure for the product that forms when boron trifluoride combines with ammonia. r...
Questions



World Languages, 14.12.2020 19:00


Biology, 14.12.2020 19:00

History, 14.12.2020 19:00

Chemistry, 14.12.2020 19:00

English, 14.12.2020 19:00

Mathematics, 14.12.2020 19:00

Mathematics, 14.12.2020 19:00

Mathematics, 14.12.2020 19:00


English, 14.12.2020 19:00





Spanish, 14.12.2020 19:00


Mathematics, 14.12.2020 19:00

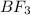 +
+ 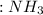 ⇒ F3
⇒ F3