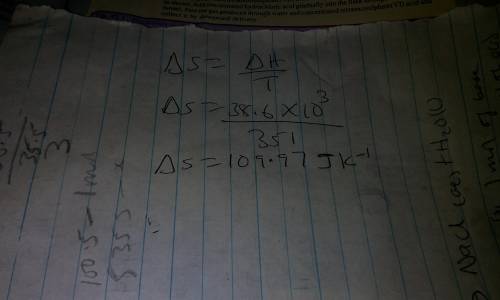Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

You know the right answer?
The boiling point of ethanol is 78 °c. the molar heat(i. e., enthalpy)of vaporization of ethanol is...
Questions



Mathematics, 08.06.2021 05:10



Mathematics, 08.06.2021 05:10





Mathematics, 08.06.2021 05:10


Biology, 08.06.2021 05:10



Mathematics, 08.06.2021 05:10


Mathematics, 08.06.2021 05:10

Social Studies, 08.06.2021 05:10