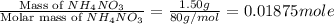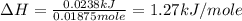A1.50 g sample of solid nh₄no₃ was added to 35.0 ml of water in a styrofoam cup (insulated from the environment) and stirred until it dissolved. the temperature of the solution dropped from 22.7°c to 19.4°c. what is the heat of reaction for dissolving nh₄no₃, expressed in kj/mol?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
A1.50 g sample of solid nh₄no₃ was added to 35.0 ml of water in a styrofoam cup (insulated from the...
Questions


History, 27.09.2019 16:30

Mathematics, 27.09.2019 16:30

Mathematics, 27.09.2019 16:30



Mathematics, 27.09.2019 16:30

Biology, 27.09.2019 16:30

Physics, 27.09.2019 16:30

Mathematics, 27.09.2019 16:30

Mathematics, 27.09.2019 16:30

Mathematics, 27.09.2019 16:30

Mathematics, 27.09.2019 16:30

Social Studies, 27.09.2019 16:30

Mathematics, 27.09.2019 16:30

English, 27.09.2019 16:30

Physics, 27.09.2019 16:30

Chemistry, 27.09.2019 16:30


Computers and Technology, 27.09.2019 16:30

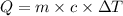
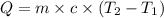
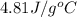
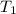 = initial temperature =
= initial temperature = 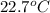
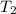 = final temperature =
= final temperature = 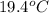
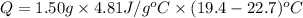
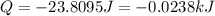
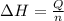
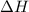 = enthalpy change = ?
= enthalpy change = ?