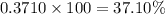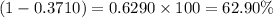Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
The element iridium exists in nature as two isotopes: 191ir has a mass of 190.9606 u, and 193ir has...
Questions

History, 20.07.2019 16:40



Mathematics, 20.07.2019 16:40







Mathematics, 20.07.2019 16:40




History, 20.07.2019 16:40


Social Studies, 20.07.2019 16:40



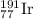 and
and 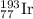 isotopes are 37.10% and 62.90% respectively.
isotopes are 37.10% and 62.90% respectively.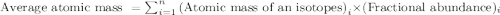 .....(1)
.....(1)![192.22=[(190.9606\times x)+(192.9629\times (1-x))]\\\\x=0.3710](/tpl/images/0466/0782/c8fc1.png)