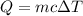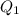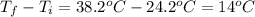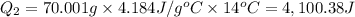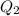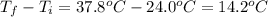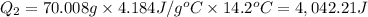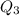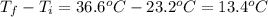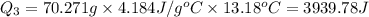Scoring scheme: 3-3-2-1 part i. for each trial, enter the amount of heat gained by the cool water, qcool water. the specific heat of water is 4.184 j/goc. report your answer to 4 digits. note: you should always carry 1 or 2 extra digits beyond the number of significant figures until your final calculation.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1
You know the right answer?
Scoring scheme: 3-3-2-1 part i. for each trial, enter the amount of heat gained by the cool water,...
Questions


Biology, 03.11.2020 14:00

Mathematics, 03.11.2020 14:00

Mathematics, 03.11.2020 14:00

Chemistry, 03.11.2020 14:00


Mathematics, 03.11.2020 14:00

Business, 03.11.2020 14:00


History, 03.11.2020 14:00



Mathematics, 03.11.2020 14:00

Mathematics, 03.11.2020 14:00


Social Studies, 03.11.2020 14:00

English, 03.11.2020 14:00

English, 03.11.2020 14:00

Business, 03.11.2020 14:00

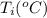 24.2, 24.0 , 23.2
24.2, 24.0 , 23.2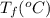 38.2, 37.8 , 36.6
38.2, 37.8 , 36.6