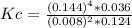Chemistry, 22.01.2020 22:31 Shamplo8817
Steam reforming of methane ( ch4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 125l tank with 20 mol of methane gas and 10 mol of water vapor at 38 degrees celsius. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of hydrogen gas to be 18 mol . calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1
You know the right answer?
Steam reforming of methane ( ch4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hy...
Questions

English, 12.07.2021 22:50











Mathematics, 12.07.2021 22:50

Mathematics, 12.07.2021 22:50


Mathematics, 12.07.2021 22:50


Health, 12.07.2021 22:50

Mathematics, 12.07.2021 22:50

Mathematics, 12.07.2021 22:50

Mathematics, 12.07.2021 22:50

![Kc = \frac{[H_2]^4*[CO_2]}{[H_2O]^2*[CH_4]}](/tpl/images/0466/2862/a2afc.png)