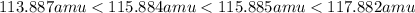The two naturally occurring isotopes of bromine are
81br (80.916 amu, 49.31%) and
79br (78.918 amu, 50.69%).
the two naturally occurring isotopes of chlorine are
37cl (36.966 amu, 24.23%) and
35cl (34.969 amu, 75.77%).
bromine and chlorine combine to form bromine monochloride, brcl.
what are the masses of the four different brcl molecules? express the masses in atomic mass units using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
what is the density of an object that has a mass of 10 g and a volumeof 5 ml? a. 0.5 g/ mlb. 2 g/mlc. 15 g/ mld. 50 g/ ml
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
The two naturally occurring isotopes of bromine are
81br (80.916 amu, 49.31%) and
<...
81br (80.916 amu, 49.31%) and
<...
Questions





Mathematics, 08.01.2020 23:31

Mathematics, 08.01.2020 23:31




Mathematics, 08.01.2020 23:31

Mathematics, 08.01.2020 23:31




Mathematics, 08.01.2020 23:31




Mathematics, 08.01.2020 23:31

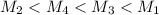
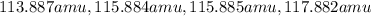
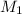 = 80.916 amu + 36.966 amu
= 80.916 amu + 36.966 amu 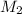 = 78.918 amu + 34.969 amu
= 78.918 amu + 34.969 amu  = 80.916 amu + 34.969 amu
= 80.916 amu + 34.969 amu 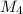 = 78.918 amu + 36.966 amu
= 78.918 amu + 36.966 amu