
Chemistry, 23.01.2020 03:31 diamondpositive68
Amixture of xenon and oxygen gas is compressed from a volume of 99.0 l to a volume of 98.0 l, while the pressure is held constant at 69.0 atm. calculate the work done on the gas mixture. be sure your answer has the correct sign (positive or negative) and the correct number of significant digits

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
Amixture of xenon and oxygen gas is compressed from a volume of 99.0 l to a volume of 98.0 l, while...
Questions

Mathematics, 05.10.2020 16:01



Mathematics, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01


English, 05.10.2020 16:01

Physics, 05.10.2020 16:01



Biology, 05.10.2020 16:01




Mathematics, 05.10.2020 16:01





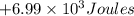
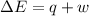
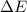 =Change in internal energy
=Change in internal energy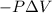 {Work done on the system is positive when the final volume is lesser than initial volume }
{Work done on the system is positive when the final volume is lesser than initial volume }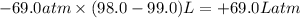
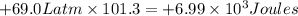 {1Latm=101.3J}
{1Latm=101.3J}

