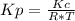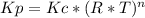Chemistry, 23.01.2020 03:31 bakaoffire
Consider the following chemical equilibrium: c(s) +2h2 (g) ch4 (g) now write an equation below that shows how to calculate from for this reaction at an absolute temperature . you can assume is comfortably above room temperature. if you include any common physical constants in your equation be sure you use their standard symbols, found in the aleks calculator.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Consider the following chemical equilibrium: c(s) +2h2 (g) ch4 (g) now write an equation below that...
Questions


Mathematics, 14.09.2021 14:00


Biology, 14.09.2021 14:00


Mathematics, 14.09.2021 14:00

Mathematics, 14.09.2021 14:00


Mathematics, 14.09.2021 14:00






Mathematics, 14.09.2021 14:00


Mathematics, 14.09.2021 14:00

Mathematics, 14.09.2021 14:00