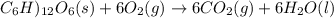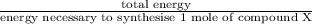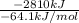Chemistry, 23.01.2020 03:31 coleman310
Assume that the complete combustion of one mole of fructose, a monosaccharide, to carbon dioxide and water liberates 2810 kj (δg°\' = –2810 kj/mol). if the energy generated by the combustion of fructose is entirely converted to the synthesis of a hypothetical compound x, calculate the number of moles of the compound that could theoretically be generated. use the value δg°\'compound x = − 64.1 kj/mol kj/mol. round your answer to two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3


Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1
You know the right answer?
Assume that the complete combustion of one mole of fructose, a monosaccharide, to carbon dioxide and...
Questions

Social Studies, 20.04.2020 21:18


Mathematics, 20.04.2020 21:18

Mathematics, 20.04.2020 21:18





Mathematics, 20.04.2020 21:18






History, 20.04.2020 21:19

English, 20.04.2020 21:19

Biology, 20.04.2020 21:19

Mathematics, 20.04.2020 21:19


History, 20.04.2020 21:19