
Chemistry, 23.01.2020 04:31 chivitogomez2400
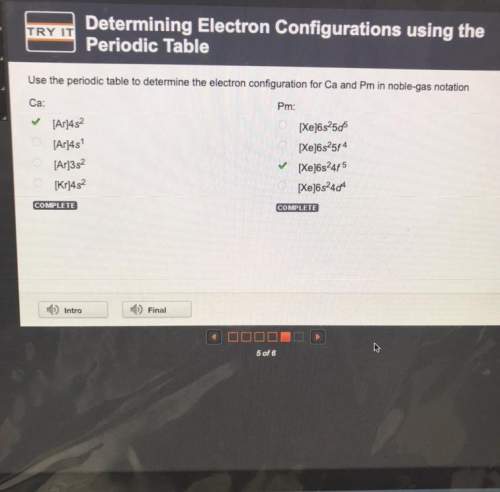
Use the periodic table to determine the electron configuration for ca and pm in noble-gas notation
ca:
pm:
✓
ca:
[ar]4s
[ar]4s
[xe]6s2506
[xe]6s25f4
pm:
[xe6s245
[xe]6s244
[ar]3s2
[kr]452

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2
You know the right answer?
Use the periodic table to determine the electron configuration for ca and pm in noble-gas notation
Questions




Mathematics, 07.07.2019 04:00




Mathematics, 07.07.2019 04:00

English, 07.07.2019 04:00

History, 07.07.2019 04:00

Social Studies, 07.07.2019 04:00

Mathematics, 07.07.2019 04:00


Biology, 07.07.2019 04:00


Physics, 07.07.2019 04:00



Social Studies, 07.07.2019 04:10



