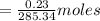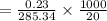Chemistry, 23.01.2020 05:31 lovexoxdivap0ifhi
Apure organic amide product (2.85 g) which has a molecular weight of 285.34 g/mol was fully dissolved in 20ml of the boiling solvent, hexanes. this organic amide product then underwent a recrystallization when the solvent was cooled to 0° c. the recrystallized organic product (2.62 grams) was obtained after vacuum filtration. what is the solubility of the organic amide product in the solvent, hexanes, at 0° c?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
Apure organic amide product (2.85 g) which has a molecular weight of 285.34 g/mol was fully dissolve...
Questions

Mathematics, 28.08.2019 15:10

Chemistry, 28.08.2019 15:10

Chemistry, 28.08.2019 15:10


Mathematics, 28.08.2019 15:10


Mathematics, 28.08.2019 15:10




Chemistry, 28.08.2019 15:10


Computers and Technology, 28.08.2019 15:10


History, 28.08.2019 15:10

Spanish, 28.08.2019 15:10

Mathematics, 28.08.2019 15:10

Mathematics, 28.08.2019 15:10

Mathematics, 28.08.2019 15:10