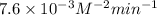Chemistry, 23.01.2020 05:31 NetherisIsTheQueen
2hgcl2(aq) + c2o42–(aq) → 2cl–(aq) +2co2(g) + hg2cl2(s)
the above reaction was studied by the method of initial rates and the following results were obtained:
[hgcl2] (m)
[c2o42–] (m)
initial rate (m/min)
0.105
0.15
1.8x10–5
0.105
0.30
7.2x10–5
0.0525
0.30
3.6 x10–5
0.0525
0.15
9.0x10–6
how would you describe the kinetics of this reaction? what is the rate constant?
first-order wrt hgcl2, second-order wrt c2o42–, third-order overall
k = 7.6x10–3 m–2min–1
first-order with respect to (wrt) hgcl2, first-order wrt c2o42–, second-order overall
k = 1.1x10–3 m–1min–1
zero-order wrt hgcl2, second-order wrt c2o42–, second-order overall
k = 5.4x10–5 mmin–1
first-order wrt hgcl2, second-order wrt c2o42–, second-order overall
k = 4.3x10–8 m–2min–1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3
You know the right answer?
2hgcl2(aq) + c2o42–(aq) → 2cl–(aq) +2co2(g) + hg2cl2(s)
the above reaction was studied b...
the above reaction was studied b...
Questions


Mathematics, 18.06.2020 00:57


Mathematics, 18.06.2020 00:57


Mathematics, 18.06.2020 00:57

Mathematics, 18.06.2020 00:57





Mathematics, 18.06.2020 00:57





Computers and Technology, 18.06.2020 00:57



![1.8 \times 10^{-5} = k[0.105]x [0.15]y](/tpl/images/0466/8825/5cde5.png) ............. (1)
............. (1)![7.2 \times 10^{-5} = k [0.105]x [0.30]y](/tpl/images/0466/8825/631f4.png) ........... (2)
........... (2)![3.6 \times 10^{-5} = k [0.0525]x [0.30]y](/tpl/images/0466/8825/aedb0.png) ............ (3)
............ (3)![k[HgCl_{2}]x [C_{2}O^{2-}_{4}]y](/tpl/images/0466/8825/d43c2.png)
![k [HgCl2]1 [C_{2}O^{-2}_{4}]2](/tpl/images/0466/8825/e7078.png)
![1.8 \times 10^{-5} = k [0.105]1 [0.15]2](/tpl/images/0466/8825/b28f1.png)