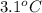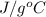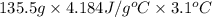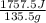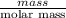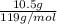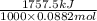Acoffee cup calorimeter initially contains 125g of water at 24.2oc. 10.5g of potassium bromide also at 24.2oc is added to the water. after the kbr dissolves the final temperature is 21.1oc. calculate the enthalpy change for dissolving the salt in j/g and kj/mol. assume specific heat of solution is 4.18j/goc.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
Acoffee cup calorimeter initially contains 125g of water at 24.2oc. 10.5g of potassium bromide also...
Questions

Mathematics, 05.02.2021 03:30



Mathematics, 05.02.2021 03:30


Business, 05.02.2021 03:30


English, 05.02.2021 03:30

Mathematics, 05.02.2021 03:30

Mathematics, 05.02.2021 03:30

Mathematics, 05.02.2021 03:30



History, 05.02.2021 03:30




Chemistry, 05.02.2021 03:30

Physics, 05.02.2021 03:30

Mathematics, 05.02.2021 03:30