
Chemistry, 23.01.2020 19:31 andrewschmitz4704
Consider two solutions, solution a and solution b. [h+] in solution a is 500 times greater than that in solution b. what is the difference in the ph values of the two solutions?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2
You know the right answer?
Consider two solutions, solution a and solution b. [h+] in solution a is 500 times greater than that...
Questions

History, 03.12.2021 01:10



Social Studies, 03.12.2021 01:10

History, 03.12.2021 01:10

Mathematics, 03.12.2021 01:10



Social Studies, 03.12.2021 01:10





Computers and Technology, 03.12.2021 01:10

Social Studies, 03.12.2021 01:10



Mathematics, 03.12.2021 01:10

History, 03.12.2021 01:10

Physics, 03.12.2021 01:10

![pH=-\log [H^+]](/tpl/images/0467/6092/37e81.png)
![[H^+]](/tpl/images/0467/6092/07acb.png) =
= 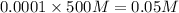
![pH=-\log[0.05]](/tpl/images/0467/6092/b675d.png)
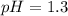

![pH=-\log[0.0001]](/tpl/images/0467/6092/e294d.png)



