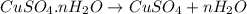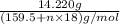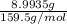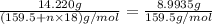Copper(ii) sulfate forms a bright blue hydrate with the formula cuso 4 ⋅ n h 2 o ( s ) . if this hydrate is heated to a high enough temperature, h 2 o ( g ) can be driven off, leaving the grey‑white anhydrous salt cuso 4 ( s ) . a 14.220 g sample of the hydrate was heated to 300 ∘ c . the resulting cuso 4 ( s ) had a mass of 8.9935 g . calculate the val

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
Copper(ii) sulfate forms a bright blue hydrate with the formula cuso 4 ⋅ n h 2 o ( s ) . if this hyd...
Questions

Mathematics, 16.07.2020 17:01




Mathematics, 16.07.2020 17:01


History, 16.07.2020 17:01

Mathematics, 16.07.2020 17:01


Mathematics, 16.07.2020 17:01


Mathematics, 16.07.2020 17:01



Chemistry, 16.07.2020 17:01



Mathematics, 16.07.2020 17:01


Mathematics, 16.07.2020 17:01

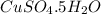 .
.