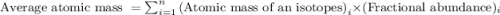Chemistry, 24.01.2020 01:31 ashkids7178
The element copper has two stable isotopes, copper-63 with a mass of 62.93 amu and copper-65 with a mass of 64.93 amu. from the atomic weight of cu = 63.54 one can conclude that:
- both isotopes have the same percent natural abundance
- copper-65 has the highest percent natural abundance
- most copper atoms have an atomic mass of 63.54
- copper-63 has the highest percent natural abundance

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
The element copper has two stable isotopes, copper-63 with a mass of 62.93 amu and copper-65 with a...
Questions




History, 08.02.2021 21:10


Mathematics, 08.02.2021 21:10






Chemistry, 08.02.2021 21:10




Mathematics, 08.02.2021 21:10


Mathematics, 08.02.2021 21:10