
Chemistry, 24.01.2020 02:31 SkirrtCrackers
For a certain chemical reaction, the standard gibbs free energy of reaction is 144. kj. calculate the temperature at which the equilibrium constant k = 5.9 × 10 . round your answer to the nearest degree.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Where are each of the three particles located within the atom?
Answers: 1


Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1
You know the right answer?
For a certain chemical reaction, the standard gibbs free energy of reaction is 144. kj. calculate th...
Questions



History, 22.10.2019 05:50

Mathematics, 22.10.2019 05:50


Biology, 22.10.2019 05:50

Mathematics, 22.10.2019 05:50

Advanced Placement (AP), 22.10.2019 05:50

Mathematics, 22.10.2019 05:50

Mathematics, 22.10.2019 05:50


Computers and Technology, 22.10.2019 05:50

History, 22.10.2019 05:50

Geography, 22.10.2019 05:50

Mathematics, 22.10.2019 05:50




Mathematics, 22.10.2019 05:50

Biology, 22.10.2019 05:50

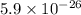 .
.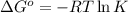
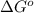 = standard Gibbs free energy = 144.0 kJ=144,000 J (Conversion factor: 1kJ = 1000J)
= standard Gibbs free energy = 144.0 kJ=144,000 J (Conversion factor: 1kJ = 1000J)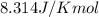
![144,000 J/mol=-(8.3145J/Kmol)\times T\times \ln [5.9\times 10^{-26}]](/tpl/images/0468/2071/84a5d.png)
![T=\frac{144,000 J/mol}{-(8.314 J/Kmol)\times \ln [5.9\times 10^{-26}]}](/tpl/images/0468/2071/493d4.png)


