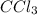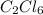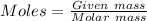Chemistry, 24.01.2020 02:31 hqlego6882
Acompound contains 10.13% c and 89.87% cl (by mass). determine both the empirical formula and the molecular formula of the compound given that the molar mass is 237 g/mol.
ccl3
c2cl
ccl

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2


Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2
You know the right answer?
Acompound contains 10.13% c and 89.87% cl (by mass). determine both the empirical formula and the mo...
Questions

Geography, 22.12.2020 20:40




History, 22.12.2020 20:40

Mathematics, 22.12.2020 20:40

Social Studies, 22.12.2020 20:40

Mathematics, 22.12.2020 20:40


Mathematics, 22.12.2020 20:40




Mathematics, 22.12.2020 20:40





Mathematics, 22.12.2020 20:40

Mathematics, 22.12.2020 20:40