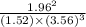Chemistry, 24.01.2020 10:31 cheervolley
A1.000 l vessel is filled with 1.000 mole of n2,2.000 moles of h2, and 3.000 moles of nh3. when the reaction n2(g) + 3 h2(g)⇀↽2 nh3(g) comes to equilibrium, it is observed that the concentration of nh3is 2.12 moles/l. what is the numerical value of the equilibrium constant kc?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1
You know the right answer?
A1.000 l vessel is filled with 1.000 mole of n2,2.000 moles of h2, and 3.000 moles of nh3. when the...
Questions



Mathematics, 17.12.2019 22:31




History, 17.12.2019 22:31


Mathematics, 17.12.2019 22:31

Social Studies, 17.12.2019 22:31


Mathematics, 17.12.2019 22:31



Chemistry, 17.12.2019 22:31

Arts, 17.12.2019 22:31

History, 17.12.2019 22:31



English, 17.12.2019 22:31

![[NH^3]= \frac{3mol}{1l}](/tpl/images/0468/7747/69ad7.png) = 3M
= 3M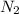 ] = 1M
] = 1M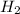 ] = 2 M
] = 2 M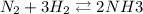
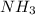 ] = 1.96 M
] = 1.96 M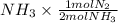 ) = 0.52 mol created (in addition to 1 mol already in vessel)
) = 0.52 mol created (in addition to 1 mol already in vessel)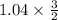 1.56 moles created
1.56 moles created  ] = 1.56 + 2 = 3.56 M
] = 1.56 + 2 = 3.56 M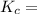
![\frac{[NH^3]^2}{N^2[H^2]^3}](/tpl/images/0468/7747/7f945.png)