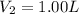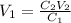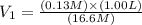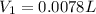Chemistry, 24.01.2020 21:31 RoyalGurl01
You wish to prepare 0.13 m hno3 from a stock solution of nitric acid that is 16.6 m. how many milliliters of the stock solution do you require to make up 1.00 l of 0.13 m hno3?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3


Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
You wish to prepare 0.13 m hno3 from a stock solution of nitric acid that is 16.6 m. how many millil...
Questions

Physics, 07.11.2019 15:31




English, 07.11.2019 15:31






Social Studies, 07.11.2019 15:31

Business, 07.11.2019 15:31

Mathematics, 07.11.2019 15:31


Biology, 07.11.2019 15:31


Mathematics, 07.11.2019 15:31

Social Studies, 07.11.2019 15:31

Mathematics, 07.11.2019 15:31

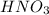
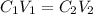
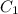 and
and 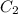 are initial and final concentration respectively.
are initial and final concentration respectively. 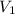 and
and  are initial and final volume respectively.
are initial and final volume respectively.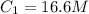 ,
, 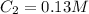 and
and