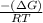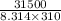Chemistry, 24.01.2020 23:31 haileyw123
Citrate synthase catalyzes the reaction: + − → + − the standard free energy change for the reaction is −31.5 ∙ −1. calculate the equilibrium constant for this reaction at 37℃.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

You know the right answer?
Citrate synthase catalyzes the reaction: + − → + − the standard free energy change for the re...
Questions

Mathematics, 31.03.2021 18:00

History, 31.03.2021 18:00


Geography, 31.03.2021 18:00

Physics, 31.03.2021 18:00

Mathematics, 31.03.2021 18:00

Mathematics, 31.03.2021 18:00

Social Studies, 31.03.2021 18:00

Social Studies, 31.03.2021 18:00




Advanced Placement (AP), 31.03.2021 18:00

Mathematics, 31.03.2021 18:00

Mathematics, 31.03.2021 18:00

Social Studies, 31.03.2021 18:00

Advanced Placement (AP), 31.03.2021 18:00


Mathematics, 31.03.2021 18:00

Mathematics, 31.03.2021 18:00

 citrate + HS-CoA
citrate + HS-CoA
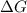 ) of a reaction and equilibrium constant (K) is as follows.
) of a reaction and equilibrium constant (K) is as follows.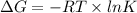
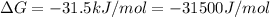 (as 1 kJ = 1000 J)
(as 1 kJ = 1000 J)