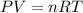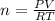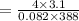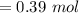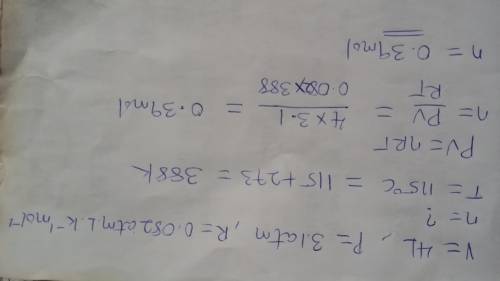Chemistry, 25.01.2020 01:31 austintules2005
In a 4.00 l pressure cooker, water is brought to a boil. if the final temperature is 115 °c at 3.10 atm, how many moles of steam are in the cooker?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
In a 4.00 l pressure cooker, water is brought to a boil. if the final temperature is 115 °c at 3.10...
Questions



History, 04.12.2020 05:30

Biology, 04.12.2020 05:30

Business, 04.12.2020 05:30

French, 04.12.2020 05:30

English, 04.12.2020 05:30

Law, 04.12.2020 05:30







Mathematics, 04.12.2020 05:30


Computers and Technology, 04.12.2020 05:30

Mathematics, 04.12.2020 05:30


Mathematics, 04.12.2020 05:30