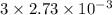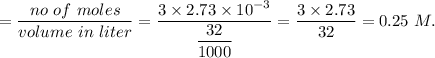Chemistry, 25.01.2020 02:31 Ayyyyeeeeeeewuzgud
A32.00 ml sample of an unknown h3po4 solution is titrated with a 0.110 m naoh solution. the equivalence point is reached when 24.83 ml of naoh solution is added. what is the concentration of the unknown h3po4 solution? the neutralization reaction is
h3po4(aq)+3naoh(aq)→3h2o(l)+na3po4( aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2
You know the right answer?
A32.00 ml sample of an unknown h3po4 solution is titrated with a 0.110 m naoh solution. the equivale...
Questions


Mathematics, 04.06.2021 02:40

Mathematics, 04.06.2021 02:40


Biology, 04.06.2021 02:40

Physics, 04.06.2021 02:40

Mathematics, 04.06.2021 02:40

Mathematics, 04.06.2021 02:40



Mathematics, 04.06.2021 02:40


Biology, 04.06.2021 02:40




Biology, 04.06.2021 02:40



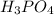 in sample is 0.25 M.
in sample is 0.25 M.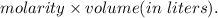
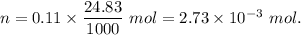
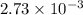 mol of NaOH reacts with
mol of NaOH reacts with