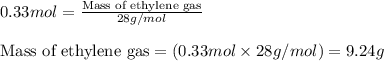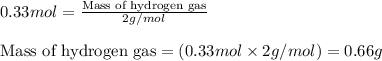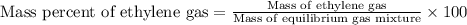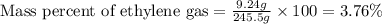Ethylene (ch2ch2) is the starting point for a wide array of industrial chemical syntheses. for example, worldwide about 8.0 x 1010kg of polyethylene are made from ethylene each year, for use in everything from household plumbing to artificial joints. natural sources of ethylene are entirely inadequate to meet world demand, so ethane (ch3ch3) from natural gas is "cracked" in refineries at high temperature in a kineticallycomplex reaction that produces ethylene gas and hydrogen gas. suppose an engineer studying ethane cracking fills a 30.0l reaction tank with 24.0atm of ethane gas and raises the temperature to 800.°c. he believes kp= 0.040 at this temperature. calculate the percent by mass of ethylene the engineer expects to find in the equilibrium gas mixture. round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3

Chemistry, 23.06.2019 14:50
Write an equation to show action of positive and negative catalyst
Answers: 1
You know the right answer?
Ethylene (ch2ch2) is the starting point for a wide array of industrial chemical syntheses. for examp...
Questions

Advanced Placement (AP), 06.09.2021 14:00

Health, 06.09.2021 14:00

Engineering, 06.09.2021 14:00


Mathematics, 06.09.2021 14:00

Mathematics, 06.09.2021 14:00


Computers and Technology, 06.09.2021 14:00


Social Studies, 06.09.2021 14:00



Chemistry, 06.09.2021 14:00

English, 06.09.2021 14:00


Mathematics, 06.09.2021 14:00

Social Studies, 06.09.2021 14:00



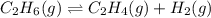
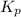 for above equation follows:
for above equation follows: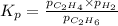
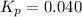
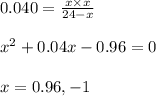
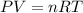 .........(1)
.........(1)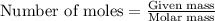 ..........(2)
..........(2)![P=23.04atm\\V=30.0L\\T=800^oC=[800+273]K=1073K\\R=0.0821\text{ L. atm }mol^{-1}K^{-1}](/tpl/images/0469/9843/5217a.png)
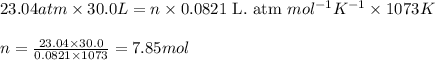
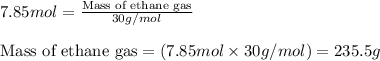
![P=0.96atm\\V=30.0L\\T=800^oC=[800+273]K=1073K\\R=0.0821\text{ L. atm }mol^{-1}K^{-1}](/tpl/images/0469/9843/9eb50.png)