
Consider the reaction 5br−(aq)+bro−3(aq)+6h+(aq)→3br2(aq) +3h2o(l) the average rate of consumption of br− is 1.76×10−4 m/s over the first two minutes. what is the average rate of formation of br2 during the same time interval? express your answer numerically in molar per second to three significant figures.
rate of formation of br2=1.14×10− 4 m/s
what is the average rate of consumption of h+ during the same time interval?
express your answer numerically in molar per second.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1
You know the right answer?
Consider the reaction 5br−(aq)+bro−3(aq)+6h+(aq)→3br2(aq) +3h2o(l) the average rate of consumption o...
Questions

Mathematics, 20.10.2019 21:30


English, 20.10.2019 21:30

Computers and Technology, 20.10.2019 21:30

English, 20.10.2019 21:30


Physics, 20.10.2019 21:30


Mathematics, 20.10.2019 21:30

Mathematics, 20.10.2019 21:30

English, 20.10.2019 21:30



Mathematics, 20.10.2019 21:30

Mathematics, 20.10.2019 21:30


Mathematics, 20.10.2019 21:30

History, 20.10.2019 21:30

Biology, 20.10.2019 21:30

Mathematics, 20.10.2019 21:30

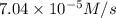 is the average rate of formation of bromine gas during the same time interval.
is the average rate of formation of bromine gas during the same time interval.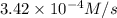 is the average rate of consumption of
is the average rate of consumption of 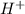 during the same time interval.
during the same time interval.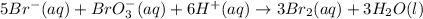
![R=-\frac{1}{5}\frac{d[Br^-]}{dt}=\frac{1}{2}\frac{d[Br_2]}{dt}](/tpl/images/0469/9513/88db1.png)
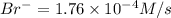
![-\frac{d[Br^-]}{dt}=1.76\times 10^{-4} M/s](/tpl/images/0469/9513/00ee7.png)
![Br_2=\frac{d[Br_2]}{dt}](/tpl/images/0469/9513/0afba.png)
![-\frac{1}{5}\frac{d[Br^-]}{dt}=\frac{1}{2}\frac{d[Br_2]}{dt}](/tpl/images/0469/9513/ad2e5.png)
![\frac{1}{5}\times 1.76\times 10^{-4} M/s=\frac{1}{2}\frac{d[Br_2]}{dt}](/tpl/images/0469/9513/05d1e.png)
![\frac{d[Br_2]}{dt}=7.04\times 10^{-5} M/s](/tpl/images/0469/9513/57d0d.png)
![R=-\frac{1}{6}\frac{d[H^+]}{dt}=\frac{1}{2}\frac{d[Br_2]}{dt}](/tpl/images/0469/9513/e620b.png)
![Br_2=\frac{d[Br_2]}{dt}=1.14\times 10^{-4} M/s](/tpl/images/0469/9513/ac1ec.png)
![H^+=-\frac{d[H^+]}{dt}](/tpl/images/0469/9513/4adf5.png)
![-\frac{1}{6}\frac{d[H^+]}{dt}=\frac{1}{2}\frac{d[Br_2]}{dt}](/tpl/images/0469/9513/5aac2.png)
![-\frac{1}{6}\frac{d[H^+]}{dt}=\frac{1}{2}\times 1.14\times 10^{-4} M/s](/tpl/images/0469/9513/ae571.png)
![-\frac{d[H^+]}{dt}=3.42\times 10^{-4} M/s](/tpl/images/0469/9513/687ef.png)


