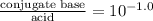Chemistry, 25.01.2020 04:31 loveoneonly9154
Calculation of molar ratios of conjugate base to weak acid from ph for a weak acid with a pka of 6.0, calculate the ratio of conjugate base to acid at a ph of 5.0.
choice of weak acid for a buffer which of these com-pounds would be the best buffer at ph 5.0: formic acid (pka 5 3.8), acetic acid (pka 5 4.76), or ethylamine (pka 5 9.0)? briefly justify your answer.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
Calculation of molar ratios of conjugate base to weak acid from ph for a weak acid with a pka of 6.0...
Questions




English, 27.07.2019 07:30


Mathematics, 27.07.2019 07:30




World Languages, 27.07.2019 07:30

Social Studies, 27.07.2019 07:30


History, 27.07.2019 07:30

Mathematics, 27.07.2019 07:30

Biology, 27.07.2019 07:30

History, 27.07.2019 07:30

Mathematics, 27.07.2019 07:30


English, 27.07.2019 07:30

Biology, 27.07.2019 07:30

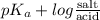
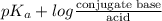
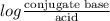 = -1.0
= -1.0