
Chemistry, 25.01.2020 06:31 jaleewoodyard1
Apharmacist–herbalist mixed 100 g lots o st. john’s wort containing the ollowing percentages o the active component hypericin: 0.3%, 0.7%, and 0.25%. calculate the percent strength o hypericin in the mixture.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1



Chemistry, 23.06.2019 16:00
Which part of the mantle is similar to the crust ? (science)
Answers: 1
You know the right answer?
Apharmacist–herbalist mixed 100 g lots o st. john’s wort containing the ollowing percentages o the a...
Questions


History, 20.08.2019 09:30


Social Studies, 20.08.2019 09:30

Social Studies, 20.08.2019 09:30


Biology, 20.08.2019 09:30



Mathematics, 20.08.2019 09:30


Business, 20.08.2019 09:30






Physics, 20.08.2019 09:30

World Languages, 20.08.2019 09:30

Mathematics, 20.08.2019 09:30

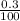 × 100
× 100 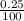 × 100
× 100 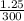 × 100
× 100 

