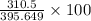Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes.
c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g)
determine the theoretical yield and the percent yields of ethanol if 735 g sucrose undergoes fermentation and 310.5 g ethanol is obtained.
theoretical
g
percent
%
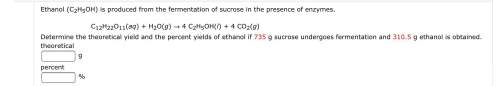

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3

Chemistry, 23.06.2019 14:00
Aracehorse with a kinetic energy of 78,750 j is clocked at 17 m/s when it crosses the finish line. what is the horse's mass?
Answers: 2

Chemistry, 23.06.2019 14:50
Write an equation to show action of positive and negative catalyst
Answers: 1
You know the right answer?
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes.
c12h...
c12h...
Questions








Chemistry, 19.03.2021 01:40


English, 19.03.2021 01:40


Mathematics, 19.03.2021 01:40

History, 19.03.2021 01:40

Mathematics, 19.03.2021 01:40

English, 19.03.2021 01:40


Computers and Technology, 19.03.2021 01:40

Business, 19.03.2021 01:40

Mathematics, 19.03.2021 01:40