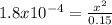Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
Calculate the ph for the following weak acid.
a solution of hcooh has 0.15m hcooh at equ...
a solution of hcooh has 0.15m hcooh at equ...
Questions

Chemistry, 11.03.2021 21:50


Physics, 11.03.2021 21:50


Mathematics, 11.03.2021 21:50

English, 11.03.2021 21:50

Mathematics, 11.03.2021 21:50





Mathematics, 11.03.2021 21:50

Social Studies, 11.03.2021 21:50

Arts, 11.03.2021 21:50


Physics, 11.03.2021 21:50

History, 11.03.2021 21:50



![ka = \frac{[H3O][HCOO]}{[HCOOH]}](/tpl/images/0471/8959/57b44.png)
![1.8 x 10^{-4} = \frac{[x][x]}{0.15 - x}](/tpl/images/0471/8959/c09c0.png)