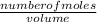Chemistry, 27.01.2020 18:31 chaitanyac90
2pb(s) + o2(aq) + 4h+(aq) → 2h2o(l) + 2pb2+(aq)
if 100 l of 0.13m lead solution is produced from the corrosion reaction, how many grams of lead metal reacted?
the answer is 0.269 g, but i don't understand the math behind getting this answer. ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

You know the right answer?
2pb(s) + o2(aq) + 4h+(aq) → 2h2o(l) + 2pb2+(aq)
if 100 l of 0.13m lead solution is produced f...
if 100 l of 0.13m lead solution is produced f...
Questions

Mathematics, 11.11.2019 18:31


Mathematics, 11.11.2019 18:31



Mathematics, 11.11.2019 18:31

Mathematics, 11.11.2019 18:31


Mathematics, 11.11.2019 18:31

English, 11.11.2019 18:31


English, 11.11.2019 18:31

Engineering, 11.11.2019 18:31


Mathematics, 11.11.2019 18:31

Mathematics, 11.11.2019 18:31


Mathematics, 11.11.2019 18:31