Chemistry, 27.01.2020 21:31 reeeeeee32
Binary compound created by reaction of an unknown element e and sulfur contains 27.52% e and 72.48% s by mass. if the formula of the compound is e4s10, calculate the atomic mass of e.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which is true of the reactants in this displacement reaction? fe + 2hcl fecl2 + h2 a. the reactants are located to the left of the arrow in the chemical equation. b. the reactants contain 1 iron atom, 2 hydrogen atoms, and 1 chlorine atom. c. the reactants are the atoms, molecules, or compounds formed in the reaction. d. the reactants have the same physical and chemical properties as the products.
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2


Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
Binary compound created by reaction of an unknown element e and sulfur contains 27.52% e and 72.48%...
Questions



Mathematics, 13.09.2019 23:30






Mathematics, 13.09.2019 23:30

Mathematics, 14.09.2019 00:10

English, 14.09.2019 00:10


English, 14.09.2019 00:10


English, 14.09.2019 00:10



History, 14.09.2019 00:10

Mathematics, 14.09.2019 00:10

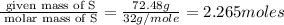
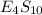
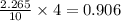 moles of E
moles of E