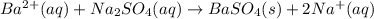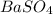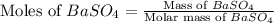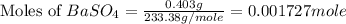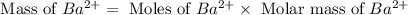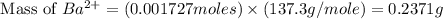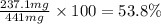Chemistry, 27.01.2020 21:31 Deliriousg636
Achemist added an excess of sodium sulfate to a solution of a soluble barium compound to precipitate all of the barium ion as barium sulfate, baso4. how many grams of barium ion are in a 441-mg sample of the barium compound if a solution of the sample gave 403 mg baso4 precipitate? what is the mass percentage of barium in the compound?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
Achemist added an excess of sodium sulfate to a solution of a soluble barium compound to precipitate...
Questions





Mathematics, 02.02.2021 02:20




Health, 02.02.2021 02:20

Chemistry, 02.02.2021 02:20

Mathematics, 02.02.2021 02:20

Mathematics, 02.02.2021 02:20




Health, 02.02.2021 02:20

Mathematics, 02.02.2021 02:20