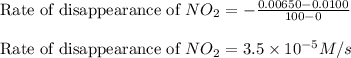Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
You know the right answer?
Nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2no2 → 2no + o2 in a parti...
Questions


Chemistry, 19.01.2021 09:40



Mathematics, 19.01.2021 09:40



English, 19.01.2021 09:40

Mathematics, 19.01.2021 09:40




Mathematics, 19.01.2021 09:40

Mathematics, 19.01.2021 09:40

Geography, 19.01.2021 09:40


Mathematics, 19.01.2021 09:40


Mathematics, 19.01.2021 09:40

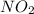 is
is 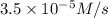
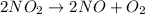
![\text{Rate of disappearance of }NO_2=-\frac{\Delta [NO_2]}{\Delta t}](/tpl/images/0473/6156/ea698.png)
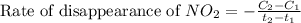
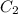 = final concentration of
= final concentration of 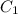 = initial concentration of
= initial concentration of  = final time = 100 minutes
= final time = 100 minutes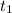 = initial time = 0 minutes
= initial time = 0 minutes