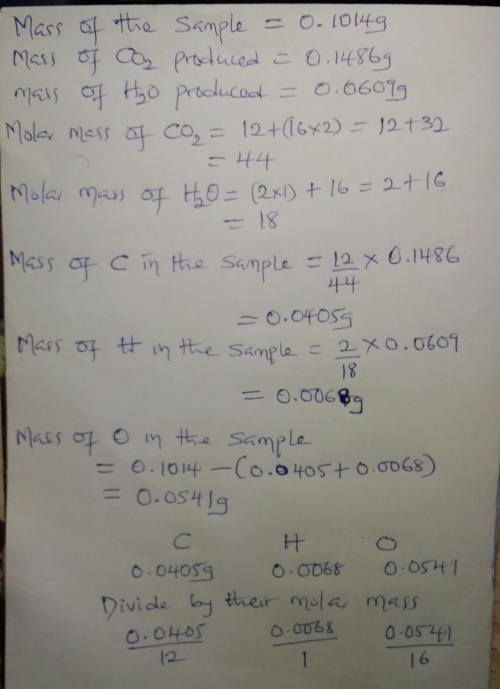Chemistry, 28.01.2020 00:31 girlygirl2007
A0.1014 g sample of a purified cho compound was burned in a combustion apparatus and produced 0.1486 g co2 and 0.0609 g of h2o. what is the empirical formula of this cho compound? enter as c#h#o#, e. g. c2h3o2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
A0.1014 g sample of a purified cho compound was burned in a combustion apparatus and produced 0.1486...
Questions


Mathematics, 26.08.2019 07:10


Mathematics, 26.08.2019 07:10

Mathematics, 26.08.2019 07:10


Business, 26.08.2019 07:10

Mathematics, 26.08.2019 07:10


Mathematics, 26.08.2019 07:10



Mathematics, 26.08.2019 07:10


History, 26.08.2019 07:10

Mathematics, 26.08.2019 07:10


Mathematics, 26.08.2019 07:10

Spanish, 26.08.2019 07:10

Mathematics, 26.08.2019 07:10