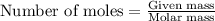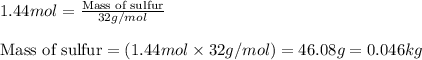Chemistry, 28.01.2020 00:31 lolfunny124
Sulfuric acid is essential to dozens of important industries from steelmaking to plastics and pharmaceuticals. more sulfuric acid is made than any other industrial chemical, and world production exceeds per year. the first step in the synthesis of sulfuric acid is usually burning solid sulfur to make sulfur dioxide gas. suppose an engineer studying this reaction introduces of solid sulfur and of oxygen gas at into an evacuated tank. the engineer believes for the reaction at this temperature. calculate the mass of solid sulfur he expects to be consumed when the reaction reaches equilibrium. round your answer to significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1

Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
You know the right answer?
Sulfuric acid is essential to dozens of important industries from steelmaking to plastics and pharma...
Questions


Mathematics, 20.11.2020 07:20







Mathematics, 20.11.2020 07:20


Social Studies, 20.11.2020 07:20

Mathematics, 20.11.2020 07:30

Mathematics, 20.11.2020 07:30


Mathematics, 20.11.2020 07:30

Law, 20.11.2020 07:30

History, 20.11.2020 07:30

Physics, 20.11.2020 07:30

Mathematics, 20.11.2020 07:30

Mathematics, 20.11.2020 07:30

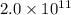 per year.
per year. 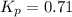 for the reaction at this temperature.
for the reaction at this temperature. 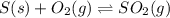
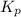 for above equation follows:
for above equation follows: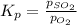
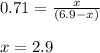
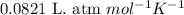
![950^oC=[950+273]K=1223K](/tpl/images/0474/1207/8397a.png)
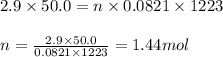
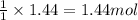 of sulfur
of sulfur