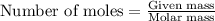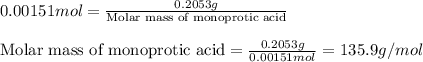Chemistry, 28.01.2020 00:31 averycipher
By titration, 15.0 ml of 0.1008 m sodium hydroxide is needed to neutralize a 0.2053-g sample of an organic acid. what is the molar mass of the acid if it is monopro-tic

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
By titration, 15.0 ml of 0.1008 m sodium hydroxide is needed to neutralize a 0.2053-g sample of an o...
Questions

History, 10.07.2019 16:00


Chemistry, 10.07.2019 16:00


Mathematics, 10.07.2019 16:00

English, 10.07.2019 16:00


History, 10.07.2019 16:00


Physics, 10.07.2019 16:00

Biology, 10.07.2019 16:00




Mathematics, 10.07.2019 16:00

Business, 10.07.2019 16:00


Mathematics, 10.07.2019 16:00


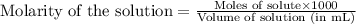
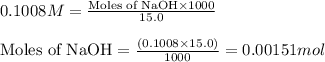
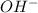 ion of NaOH neutralizes 1 mole of
ion of NaOH neutralizes 1 mole of 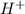 ion of monoprotic acid
ion of monoprotic acid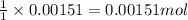 of
of