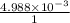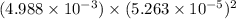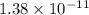Chemistry, 28.01.2020 01:31 SchoolSucks234
The solubility product of calcium fluoride (caf2(s); fluorite) is 310-11 at 25c. could a fluoride concentration of 1.0 mg l-1 be obtained in water that contains 200 mg l-1 of calcium?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
The solubility product of calcium fluoride (caf2(s); fluorite) is 310-11 at 25c. could a fluoride...
Questions





Mathematics, 12.02.2021 17:50

Mathematics, 12.02.2021 17:50

Social Studies, 12.02.2021 17:50

Mathematics, 12.02.2021 17:50



Mathematics, 12.02.2021 17:50

Mathematics, 12.02.2021 17:50


Mathematics, 12.02.2021 17:50



Mathematics, 12.02.2021 17:50



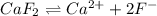
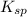 will be as follows.
will be as follows.![K_{sp} = [Ca^{2+}][F^{-}]^{2}](/tpl/images/0474/4010/1d9c8.png)
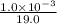
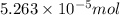
![[F^{-}] = \frac{\text{moles of F^{-}}{volume}](/tpl/images/0474/4010/e0719.png)
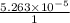
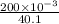
![[Ca^{2+}] = \frac{moles of Ca^{2+}}{volume}](/tpl/images/0474/4010/ce6ee.png)