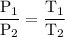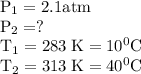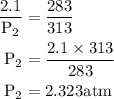Chemistry, 28.01.2020 01:31 datboyjulio21
At 10.°c, 20.g of oxygen gas exerts a pressure of 2.1atm in a rigid, 7.0l cylinder. assuming ideal behavior, if the temperature of the gas was raised to 40.°c, which statement indicates the new pressure and explains why?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1
You know the right answer?
At 10.°c, 20.g of oxygen gas exerts a pressure of 2.1atm in a rigid, 7.0l cylinder. assuming ideal b...
Questions

Arts, 10.12.2020 19:00




Mathematics, 10.12.2020 19:00

Mathematics, 10.12.2020 19:00



Mathematics, 10.12.2020 19:00




History, 10.12.2020 19:00

Mathematics, 10.12.2020 19:00

Chemistry, 10.12.2020 19:00

Mathematics, 10.12.2020 19:00


Mathematics, 10.12.2020 19:00


 = 2.323 atm.
= 2.323 atm.  or temperature of oxygen = 10-degrees celciusPressure or P
or temperature of oxygen = 10-degrees celciusPressure or P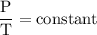 Likewise,
Likewise,