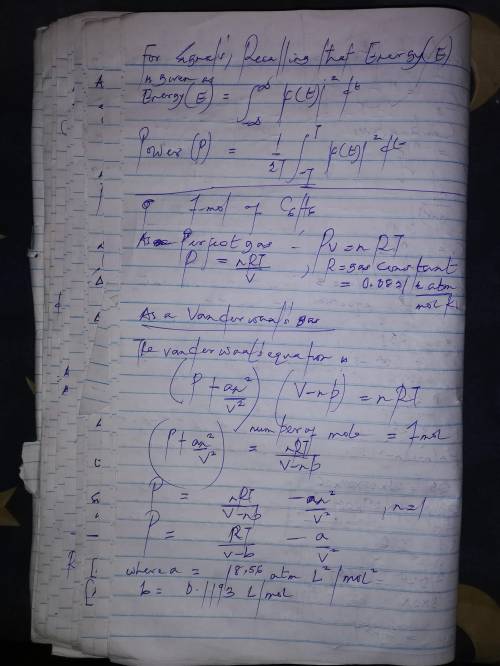Chemistry, 28.01.2020 02:31 Desinfektionsmittel
Calculate the pressure exerted by 1.0 mol of c6h6behaving as a)a perfect gas and b) a van der waals gas when it is confined under the following conditions: i)373.15 k in 22.414 dm3ii)1000 k in 22.414 dm3iii)1000 k in 150.000 dm3at which one of these conditions does the real gas be?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2


Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Calculate the pressure exerted by 1.0 mol of c6h6behaving as a)a perfect gas and b) a van der waals...
Questions

Mathematics, 27.10.2020 21:00


Geography, 27.10.2020 21:00

Mathematics, 27.10.2020 21:00


Mathematics, 27.10.2020 21:00

History, 27.10.2020 21:00



Mathematics, 27.10.2020 21:00

Mathematics, 27.10.2020 21:00

Biology, 27.10.2020 21:00

History, 27.10.2020 21:00


Mathematics, 27.10.2020 21:00


Mathematics, 27.10.2020 21:00

Computers and Technology, 27.10.2020 21:00

Social Studies, 27.10.2020 21:00