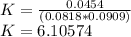Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
Asolution is made by mixing 30.0 ml of 0.150 m compound a with 25.0 ml of 0.200 m compound b. at equ...
Questions

Social Studies, 09.11.2019 04:31


English, 09.11.2019 04:31


English, 09.11.2019 04:31


Health, 09.11.2019 04:31

Mathematics, 09.11.2019 04:31


English, 09.11.2019 04:31

History, 09.11.2019 04:31


History, 09.11.2019 04:31

English, 09.11.2019 04:31



Mathematics, 09.11.2019 04:31


Biology, 09.11.2019 04:31

History, 09.11.2019 04:31

![K=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0474/5763/91a0a.png)
![K=\frac{[C]^}{[A][B]}](/tpl/images/0474/5763/1e2ca.png)
![[A]=\frac{(0.030L)*(0.150)}{0.03+0.025}=0.0818M](/tpl/images/0474/5763/9dcbf.png)
![[B]=\frac{(0.025L)*(0.200)}{0.03+0.025}=0.0909M](/tpl/images/0474/5763/9e9a1.png)
![[C]=0.0454 M](/tpl/images/0474/5763/82746.png)