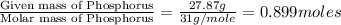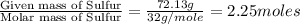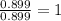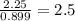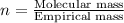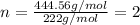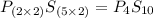Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1
You know the right answer?
Ferrophosphorus (fe2p) reacts with pyrite (fes2), producing iron (ii) sulfide and a compound that is...
Questions

History, 23.10.2021 14:00


Business, 23.10.2021 14:00

English, 23.10.2021 14:00




Mathematics, 23.10.2021 14:00



Biology, 23.10.2021 14:00


Mathematics, 23.10.2021 14:00

Arts, 23.10.2021 14:00


Mathematics, 23.10.2021 14:00

Mathematics, 23.10.2021 14:00

Mathematics, 23.10.2021 14:00

Mathematics, 23.10.2021 14:00

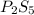 and
and 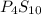 respectively.
respectively.