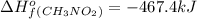Top fuel dragsters and funny cars burn nitro-methane as fuel according to the following balanced combustion equation: 2ch3no2(l)+3/2o2(g)→2co2(g)+3h2o(g) +n2(g). the standard enthalpy of combustion for nitromethane is −709.2kj/mol. calculate the standard enthalpy of formation(delta h formation) for nitro-methane.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1


Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
Top fuel dragsters and funny cars burn nitro-methane as fuel according to the following balanced com...
Questions

History, 05.11.2019 10:31

Mathematics, 05.11.2019 10:31

Mathematics, 05.11.2019 10:31


Physics, 05.11.2019 10:31



Health, 05.11.2019 10:31

Mathematics, 05.11.2019 10:31

Chemistry, 05.11.2019 10:31



History, 05.11.2019 10:31


Mathematics, 05.11.2019 10:31

Mathematics, 05.11.2019 10:31

Health, 05.11.2019 10:31

History, 05.11.2019 10:31


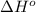
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0475/2966/45485.png)
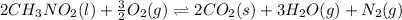
![\Delta H^o_{rxn}=[(n_{(CO_2)}\times \Delta H^o_f_{(CO_2)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})+(n_{(N_2)}\times \Delta H^o_f_{(N_2)})]-[(n_{(CH_3NO_2)}\times \Delta H^o_f_{(CH_3NO_2)})+(n_{(O_2)}\times \Delta H^o_f_{(O_2)})]](/tpl/images/0475/2966/ea72d.png)
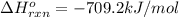
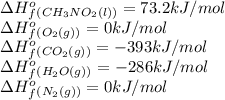
![-709.2=[(2\times -393)+(3\times -286)+(1\times 0)]-[(2\times \Delta H^o_f_{(CH_3NO_2)})+(\frac{3}{2}\times 0)]](/tpl/images/0475/2966/4d16f.png)